‘What a load of hot air!’ Parents slam Jill Biden for claiming her husband Joe is ‘working around the clock’ to end baby formula disaster as Abbott’s factory is STILL closed and foreign products not shipped in
Outraged parents are slamming First Lady Jill Biden for her patronizing public service announcement defending her husband’s response to the nationwide baby formula shortage.
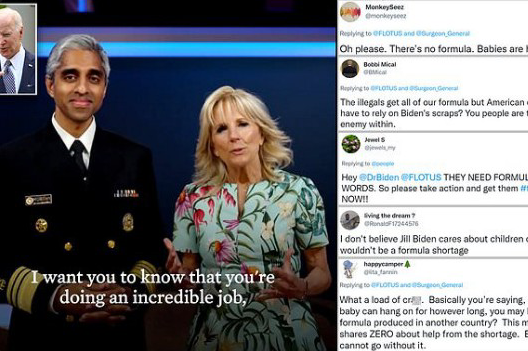
She claimed President Joe Biden is ‘working around the clock’ to get concerned parents the products they need and then applauded caregivers for ‘doing an incredible job.’
‘Becoming a mom or dad means falling in love deeper than you ever thought possible and in those first few months of sleepless nights, of endless diapers and dirty dishes…and worrying about every little danger, your love can feel like the only thing that keeps you going,’ Dr. Biden, 70, said in Tuesday’s PSA.
‘I want you to know that you’re doing an incredible job—even if you don’t always feel that way. And I know you are worried about how you are going to feed your baby. The President sees you, he hears you, and his team is working around the clock to get you what you need.’
The First Lady’s message – which comes just days after her predecessor, Melania Trump, slammed the Biden Administration’s response to the crisis – was met with heavy criticisms on Twitter from frantic mothers and fathers wade through empty grocery shelves in a struggle to find food for their babies.
‘What a load of c**p. Basically you’re saying, if your baby can hang on for however long, you may have formula produced in another country?’ one concerned citizen penned. ‘This message shares ZERO about help from the shortage. Babies cannot go without it.’
The formula shortage was caused by ongoing supply disruptions and exacerbated by a February safety recall at the nation’s largest formula manufacturing plant.
Abbott Laboratories revealed Monday that it had entered into a consent decree with the FDA that creates a pathway to reopen its Michigan baby formula factory, however the timeline for operations remains unclear.
Republicans and Democrats are introducing legislation this week that would ease the shortage and help ensure the safety and prompt delivery of baby formula to American communities for at least six months.
Dr. Biden, alongside Surgeon General Dr. Vivek Murthy, used the PSA to offer advice to parents who are struggling to find infant formula, such as using a brand that is different ‘than the one you’re used to.’
‘I know that you have questions. Any parent would,’ she said. ‘So, call your pediatrician. They can provide you the best, most updated advice. And share this with your friends, to help them as well.’
Murthy, who claimed to be impacted firsthand by the crisis, echoed her sentiment and encouraged parents to try available products, while also acknowledging that ‘change is scary’.
‘I know that can feel like a big change and be scary, but all infant formula on the shelf is safe for your baby,’ he said. ‘All formula will meet the FDA’s gold standard and only safe formula will come to American shelves.’
The pair concluded by applauded President Biden’s efforts to curb the crisis and reiterating that he is on the side of parents.
‘You should know that the president has been working tirelessly to increase the supply. He’s worked with manufacturers to increase their production capacity,’ Murthy said.
‘He’s also worked those same manufacturers and the FDA to get the closed factory back online with strict safety standards. And finally, he’s worked with the FDA and partners to import supply from abroad safely.’
Biden added: ‘Most of all, know that we’re here for you and you’re not alone.’
Unfortunately, the American people did not respond kindly to the First Lady’s pro-Joe campaign, with many critics arguing an uplifting message does nothing to help feed their children.
‘Hey @DrBiden @FLOTUS THEY NEED FORMULA NOT WORDS,’ Twitter user @jewels_my penned. ‘So please take action and get them #formula NOW!!’
‘Oh please. There’s no formula. Babies are hungry!’ echoed @monkeyseez.
User @AnnieSong62 questioned: ‘WTAF does that have to do with infant formula TROLL?’
Others issued more personal attacks against Dr. Biden, implying her message is blind to the struggles of parents.
‘You should know that the president has been working tirelessly to increase the supply. He’s worked with manufacturers to increase their production capacity,’ Murthy said.
‘He’s also worked those same manufacturers and the FDA to get the closed factory back online with strict safety standards. And finally, he’s worked with the FDA and partners to import supply from abroad safely.’
Biden added: ‘Most of all, know that we’re here for you and you’re not alone.’
Unfortunately, the American people did not respond kindly to the First Lady’s pro-Joe campaign, with many critics arguing an uplifting message does nothing to help feed their children.
‘Hey @DrBiden @FLOTUS THEY NEED FORMULA NOT WORDS,’ Twitter user @jewels_my penned. ‘So please take action and get them #formula NOW!!’
‘Oh please. There’s no formula. Babies are hungry!’ echoed @monkeyseez.
User @AnnieSong62 questioned: ‘WTAF does that have to do with infant formula TROLL?’
Others issued more personal attacks against Dr. Biden, implying her message is blind to the struggles of parents.
‘Last week, I received text messages and photos from Border Patrol agents showing a stockpile of baby formula and there was complete outrage from the White House, CNN and other liberal outlets claiming that we were lying, so I decided to go down myself and film it,’ she told Fox Business on Monday.
‘Lo and behold, not only was there stocked warehouses, but there were multiple stocked warehouses that have been not only filled with baby formula, diapers, wipes and clothing, but they have been doing this for months and there’s more en route.’
The White House, on Friday, defended the full stock of supplies, alleging Border Patrol is ‘following the law’ that requires the government to provide adequate food, specifically formula for children under the age of one, who are detained at the border.
Meanwhile, Democratic Representative Rosa DeLauro, chairwoman of the House Appropriations Committee, introduced on Tuesday legislation that would give $28 million emergency funding to the FDA to address the crisis.
She called the shortage an ‘all-hands-on-deck emergency situation.’
‘The stories of mothers and fathers struggling to find formula and the images of empty store shelves are heartbreaking,’ DeLauro said in a Tuesday statement on her new legislation.
‘Parents and caretakers across the country cannot wait—they need our support now,’ she added. ‘This bill takes important steps to restore supply in a safe and secure manner. Additionally, with these funds, FDA will be able to help to prevent this issue from occurring again.’
Congress is holding hearings this month on the infant formula shortages, and DeLauro says she is ‘eager’ to ‘find answers as to how this food safety failure occurred’ and prevent it from happening again.
‘[Th]is bill is the first step to help restock shelves and end this shortage,’ the Connecticut congresswoman noted.
Utah Republican Senator Mike Lee is also working on legislation that would help ensure the safety and prompt delivery of baby formula to American communities for at least six months.
The Fixing Our Regulatory Mayhem Upsetting Little Americans bill – or FORMULA Act – would grant a six-month waiver for tariffs on infant formula coming from other countries.
It would also grant a waiver on FDA regulations for the same time period, allowing for expedited processes to get it into the hands of parents.
‘American babies are going hungry and the federal government is standing in the way,’ Lee said in a statement on his upcoming legislative proposal in the upper chamber.
‘Current policies, tariffs, quotas, bans, and regulations are preventing mothers and fathers from being able to make the best choices to feed their babies.’
Lee’s legislation would allow those on the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) to purchase alternate, imported brands of formula for the next six months.
‘My FORMULA Act will give these families relief during this unprecedented shortage. Congress needs to pass this bill immediately to protect American babies from going hungry,’ Lee said of his bill.
Both parties are expressing the urgency needed to address the crisis.
MELANIA TRUMP SLAMS BIDEN OVER FORMULA CRISIS
Melania Trump described the shortage of baby formula nationwide as ‘heartbreaking’ – using her first interview since leaving the White House to say the crisis was down to ‘leadership’.
The former first lady sat down with Fox News’s Pete Hegseth for an interview that aired on Sunday.
Melania Trump strongly criticized Joe Biden for his handling of the baby formula crisis
‘It’s heartbreaking to see that they are struggling and the food is not available for children in 21st century in the United States of America,’ she said.
Asked what was causing the shortages, took aim at Joe Biden’s administration and replied: ‘Leadership.’
When Hegseth asked if she meant a lack of leadership, she said: ‘Yeah.’
Congress is concerned with getting shelves restocked, but are also on a mission to get to the bottom of the events leading to the shortage – a February recall of Abbott Laboratories Similac baby formulas after two infants exposed to the powdered baby formula died.
Abbott Nutrition, which operates the plant, said last week that ‘after a thorough review of all available data, there is no evidence to link our formulas to these infant illnesses.’
On Monday, the manufacturer revealed it has reached a deal with the FDA that could see it reopen its shuttered Michigan plant within two weeks, pending approval from the federal regulator.
‘After FDA approval, Abbott could restart the site within two weeks; from the time of restart it would take six to eight weeks before product is available on shelves,’ a company spokesperson told DailyMail.com on Monday.
The federal regulator, during a media call Monday night, confirmed it has not yet given its approval, saying: ‘We are negotiating with Abbott to get them up and running as soon as possible.’
The agency has declined to answer questions about the timeline for the Michigan’s plants reopening and instead directed reporters to Abbott.
‘I think we all know the treachery of giving exact timeframes to get these things done because as corrections are made sometimes new things are discovered and sometimes it goes very quickly,’ FDA Commissioner Robert Califf said. ‘Abbott itself has made a statement that they believe they could be started up within about two weeks and then up to full capacity in about two months, I think they said. You can refer back to them for details on this.’
Califf did add that he believes Abbott’s ‘timeframes are reasonable.’
The FDA, in an effort to curb the shortage, has also issued a temporary measure streamlining the importation of foreign-produced baby formula.
‘The guidance that we announced today is for 180 days, so it is a temporary measure,’ Susan Payne, Director of the FDA Center for Food Safety and Applied Nutrition, said during Monday night’s media call.
She explained that the process allows for more flexibility for foreign and domestic formula producers while the nation is currently ‘under stress’.
‘There are some differences in our regulations with regard to things like nutrition between our products and those that are sold abroad,’ Payne shared. ‘Some of that comes right from our statutes where the FDA is required to take into account certain types of factors – like the ability of these formulas to support growth and data supporting growth, growth monitoring studies.’
‘We do have certain criteria that we take into account but in this period where we are under stress we will certainly look at products that may not necessarily have the same types of data we would use in our notifications process, but have a history of safe use in other countries and do support growth.’
The $4 billion US baby formula market is dominated by domestic producers, with import options limited, subject to high tariffs and onerous safety rules that include labeling standards.
These longstanding rules have exacerbated the current crisis – and are central to officials’ efforts to ease the shortage.
Califf cautioned that foreign products are labeled with instructions written in languages that American mothers and caretakers may not understand.
‘We also have to make sure we’re testing the formula,’ he said.
TIMELINE SHOWS HOW AMERICA’S LARGEST BABY FORMULA PLANT CEASED PRODUCTION
Abbott Laboratories, the biggest baby formula supplier in the U.S., ceased production at its Michigan plant in February 2022 amid reports of fatal bacterial infections.
A timeline of events shows reveals the shut down was the plant had previously been under scrutiny by the U.S. Food and Drug Administration (FDA).
September 2021: The FDA conducted a four-day inspection of the Abbott Laboratories plant in Sturgis, Michigan.
The inspection report revealed the plant ‘did not maintain’ clean and sanitary conditions in at least one building that manufactured, processed, packaged or held baby formula.
FDA officials also observed poor hand washing among Abbott plant staff who ‘worked directly with infant formula.’
The FDA also noted an instance of improper equipment maintenance and temperature control.
October 2021: A whistleblower sends the FDA a 34-page document outlining potential concerns with the Sturgis plant.
The document, which was made public by Congresswoman Rosa DeLauro in April 2022, was written by a former plant employee.
The employee accused the plant of lax cleaning practices, falsifying records, releasing untested infant formula, and hiding information during an FDA audit in 2019, among other issues.
January – March 2022: The FDA conducted multiple inspections at the Sturgis plant over the course of three months in 2022. A ten-page inspection report revealed multiple violations at the facility.
The agency alleged the plant failed to ensure that all surfaces that contact infant formula were maintained to prevent cross-contamination.
The report states the facility ‘did not establish a system of process controls’ to ensure the baby formula ‘does not become adulterated due to the presence of microorganisms in the formula or the processing environment.’
Officials also alleged the plant failed to disclose in an investigation report whether a health hazard existed at the facility.
Additionally, the report stated plant workers were did not wear the ‘necessary protective material’ when working directly with infant formula.
February 17: U.S. health officials urgently warn parents against using three popular baby formulas manufactured at the Abbott plant in Michigan. Investigators claim the products were recently linked to bacterial contamination after an infant died and three others fell ill.
Abbott voluntarily recalled several major brands and shut down its Sturgis plant.
The FDA also said it is investigating four reports of infants who were hospitalized after consuming the formula, including one who died.
February 28: Abbott Laboratories expanded its recall of Similac baby formulas after a second infant who was exposed to the powdered baby formula died.
April 15: Abbott releases a statement alleging it is working closely with the FDA to restart operations at the Sturgis plant.
Week of April 24: The nationwide share of out-of-stock baby formula hit 40 percent. Texas, Tennessee, Missouri, Iowa, North Dakota and South Dakota, seemingly hardest hit by the shortages, reported out-of-stock rates of about 50 percent.
May 10: Abbott releases a statement to DailyMail.com claiming ‘thorough investigation’ by the FDA and Abbott revealed ‘infant formula produced at our Sturgis facility is not the likely source of infection in the reported cases and that there was not an outbreak caused by products from the facility’.
Abbott claims they are ‘working closely with the FDA to restart operations’ at the plant, with the spokesperson noting: ‘We continue to make progress on corrective actions and will be implementing additional actions as we work toward addressing items related to the recent recall’.
The FDA told DailyMail.com it was holding discussions with ‘Abbott and other manufacturers to increase production of different specialty and metabolic products’ but refused to say when the Sturgis plant could reopen.
Sen. Mitt Romney issued a letter to the FDA and U.S. Department of Agriculture (USDA) urging leaders to address the formula shortage and work to prevent future threats to infant health.
May 11: Lawmakers on Capitol Hill announce plans to hold a hearing in two weeks on infant formula shortages.
Abbott announced it would take up to ten weeks for the company to get baby formula to retailers once the Sturgis plant reopens.
Abbott also said: ‘After a thorough review of all available data, there is no evidence to link our formulas to these infant illnesses.’
May 12: White House Press Secretary Jen Psaki defends the government’s closure of the Abbott plant.
President Joe Biden met with executives from infant formula manufactures and retailers to address the shortage.
May 13: Biden addresses the formula crisis during a press briefing, saying: ‘We’re going to be, in a matter of weeks or less, getting significantly more formula on shelves.’
The FDA announced it was working to streamline a process that will get more products to consumers – while also meeting safety, quality and labeling standards
May 16: Abbott and the FDA reach agreement to reopen baby formula facility in Michigan.
However, the FDA has yet to disclose a timeframe for allowing the plant to resume production.
The FDA also implemented new measures, in effect for 180 days, to increase imports of baby formula produced overseas.
* Article from: dailymail.co.uk


